CMF Spinalogic
CMF OL1000 Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture.
Call for more Information 1-888-300-0063
CMF SpinaLogic is a portable, battery-powered, micro-controlled, noninvasive bone growth stimulator indicated as an adjunct electromagnetic treatment to primary lumbar spinal fusion surgery for one or two levels.
Features:
- Lightweight and comfortable
- Easy-to-use & Noninvasive
- Requires simple, one-button operation
- Device is worn for 30 minutes per day
- Can be used with internal or external fixation or over a cast
This treatment has been shown in pre-clinical studies to help the body's own healing process begin working. Clinical studies have shown an increased chance of healing of 60.7% in patients with nonunions that averaged 29.3 months from injury. Registry data on over 2300 patients reports a heal rate of 75.1%.

Scientific evidence supporting CMF is often considered the MOST relevant…
After testing multiple hypotheses, researcher determined that maximum bone cell response occurred within frequencies similar to those generated intrinsically by functional activity.
The noted, “Resorption of bone is lowest and new-bone formation is greatest when the power of the induced electric fields is concentrated in the very low-frequency range”
( 15Hz -150Hz).5
Further research in various frequencies impact on bone cell response showed the optimal frequency for bone healing to be 76.6Hz, the frequency now offered by the CMF bone growth stimulator system.6
Just 30 minutes exposure to 76.6Hz increased the volume and number of IGF II molecules and receptors. An increase in both have been correlated to an amplified increase in bone cell proliferation.
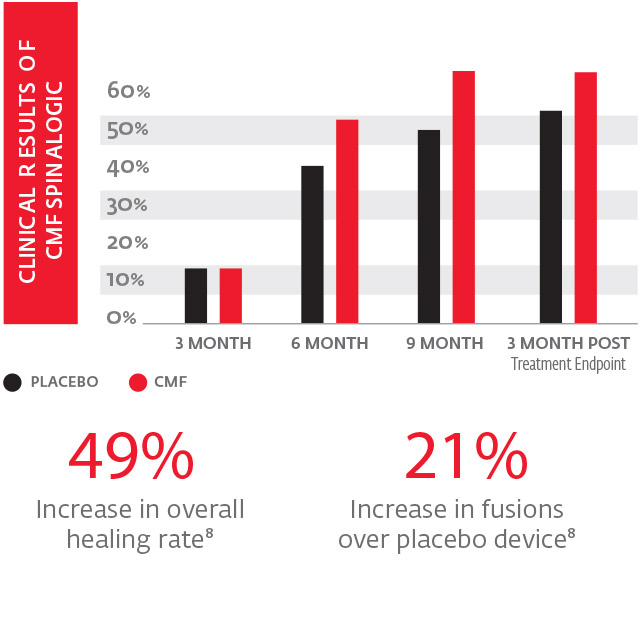
CMF Spinalogic has been proven to accelerate spinal fusion
CMF Technology is the MOST clinically effective electrical bone growth stimulator at 30 minutes per day.7
The ONLY technology proven to accelerate spine fusion in one simple, 30 minute treatment per day.
With just one 30 minute treatment per day, the CMF Spinalogic provided:

The device has the following features:
• Lightweight and comfortable
• Easy-to-use & Noninvasive
• Requires simple, one-button operation
• Device is worn for only 30 minutes per day
• Can be used with internal or external fixation or over a cast
OrthoMed Policies are the Best In The Industry!
Fast Shipping, 30 Day Money Back on most items, and Easy Returns. Please note, some Exclusions and Conditions Apply for certain products. For full policy details, including exclusions and conditions: View Our Shipping Options and Policies
Contraindications for CMF Spinalogic:
Demand-type pacemaker and implantable cardiovertor defibrillator (ICD) operation may beadversely affected by exposure to combined static and dynamic magnetic fields. Physicians should not prescribe CMF SpinaLogic for patients with such devices. The safety and effectiveness of CMF SpinaLogic in pregnant women have not been studied, and the effects of the device on the mother or the developing fetus are unknown. Thus, this device should not be used in pregnant women. If a woman becomes pregnant during treatment with CMF SpinaLogic, treatment should be discontinued immediately.
References:
- Based on comparison of FDA approval dates for all electrical stimulators.
- Based on and compared to all evidence supporting 30 minutes CMF weartime.
- CMF OL1000 is the only electrical stimulation technology indicated for 30 minutes use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones.
- Dates represent first FDA approval of technology – technology may have been approved for other products at later dates. ⁵McLeod, K.J., Rubin, C.T., The Effect of Low Frequency Electrical Fields on Osteogenesis. J. Bone Joint Surg., 74A: 920 – 929, 1992.
- Ryaby, J.T., et al., The Role of Insulin-like Growth Factor in Magnetic Field Regulation of Bone Formation, Bioelectrochemistry and Bioenergetics, 35: 87-91, 1994.
- The only technology approved and with clinical evidence at 30 minutes per day.
- Linovitz R, Pathria M, Bernhardt M, et al. Combined Magnetic Fields Accelerate and Increase Spine Fusion: A Double-Blind, Randomized, Placebo Controlled Study, Spine. 2002 July; 27(13):1383-1388. ⁹NASS Coverage Policy Recommendations for Electrical Bone Growth Stimulators, 2016.








